ATOMIC THEORY: from 440 B.C. to present
I. 440 B.C. : Leucippus and Democritus
A. All matter is composed of atoms....These atoms cannot be further split into smaller portions.
B. There is a void, which is empty space between atoms.
C. Atoms are completely solid.
D. Atoms are homogeneous, with no internal structure.
E. Atoms are different in their sizes, their shapes, and their weight.
II. 1766 - 1844 : John Dalton ďThe Father of Atomic TheoryĒ
Four basic ideas in Daltonís atomic theory:
A. Chemical elements are made of ______________
Atoms are ____________, ___________________ particles
B. The atoms of an element are _________________ in their masses
Zn =
Zn
= Zn
C. Atoms of different elements have different masses
_____ LIGHTEST _____ HEAVIER
_____ VERY HEAVY
D. Atoms only combine in small, _____________________
Law of Definite Proportions
Observation: Every time we make zinc chloride, the ratio comes out to
_________________________
- or -
0.92 g Zn
1.00 g Cl
If all ... Zn atoms have a mass of 65 units
and all ... Cl atoms have a mass of 35 units
Then, 1 atom Zn = _______
= _______
1 atom Cl
But ...
We got ... 0.92 g Zn
1.00 g Zn
Conclusion: _____________________________________
1 atom Zn = ________ = ________
2 atoms Cl
= ZnCl2 = white substance
Daltonís Atomic Model
Two of these (Daltonís items A - D) are now known to be wrong ______ and ______
III. 1856 - 1940 John Joseph (J. J.) Thomson
Thomsonís Cathode-Ray Tube
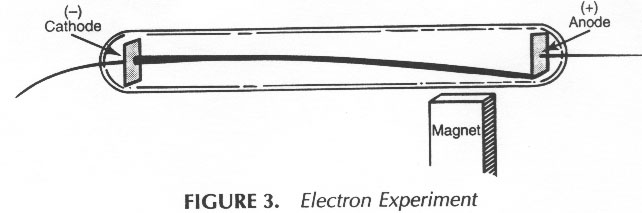 |
Observations:
1) Something went across the tube. Was it ____________ or
_____________ ?
2) Put in a pin wheel: found that it ____________
3) Easily ______________ by a ________________
4) With different ______________ or different ______________: always the same results
5) Light rays were deflected toward the _____________
Conclusions:
1) ________________________________________________________________
__________________________________________________________________
2) These cathode ray particles are ___________________ charged.
These particles were
later named _______________________.
3)___________________________________________________________________________________________________________________________________
4) Thomson used magnetic and electric fields to measure the ratio of
the electron's mass to its charge. He estimated that the
electron's
mass was ______________ the mass of the hydrogen atom.
Thomsonís Atomic Model
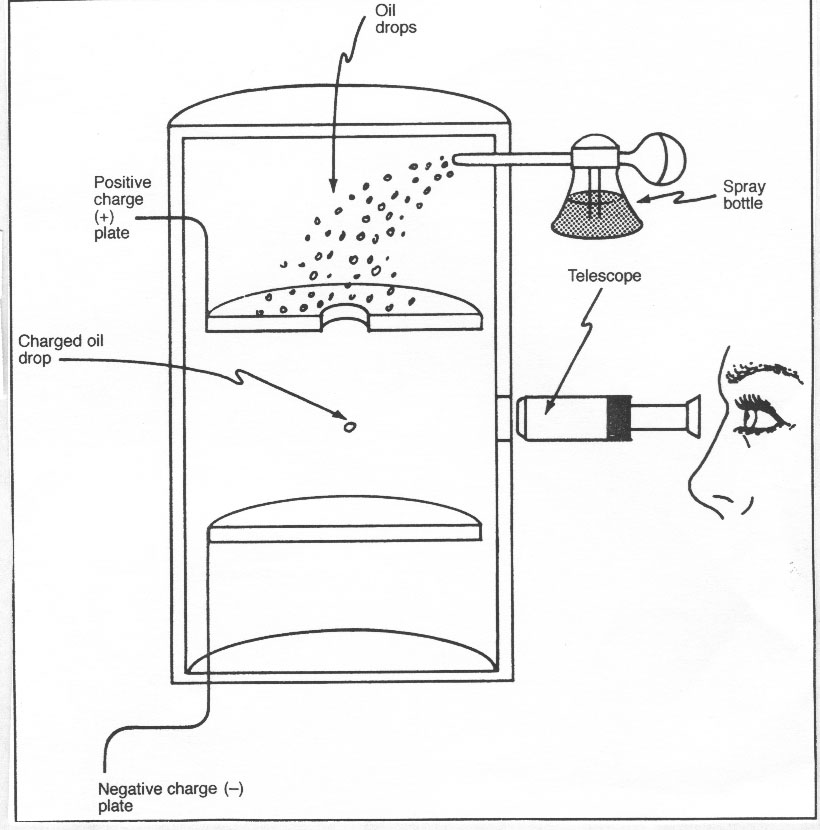
IV. 1868 - 1953 Robert Millikan
Millikanís Oil Drop Experiment
Conclusion:
1) The ____________ on an electron is _____________
2) The _________ of an electron is _____________
Va. ~ 1896 Eugene Goldstein
1) modified the cathode ray tube used by Thomson:
____________________________
___________________________________________________________________
2) observed rays traveling in the direction opposite to that of
the cathode rays:
_____________ _____________
3) concluded that they were composed of _______________
________________
4) the positive particles had a charge opposite and equal to the
negatively charged particles
of Thomson's experiments (atoms that had lost electrons and become
ions)
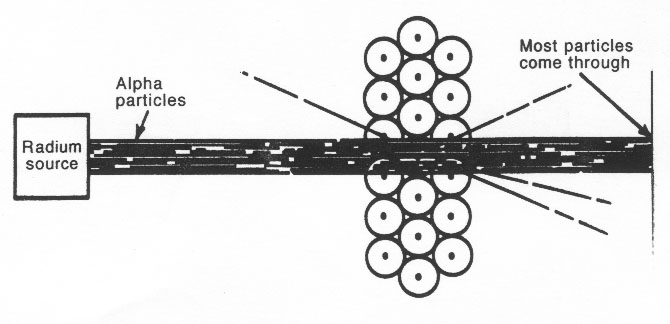
V. 1871 - 1937 Ernest Rutherford
To test the Thomson model of the atom, __________________ did a famous experiment:
Rutherfordís Gold Foil Experiment
Observations:
1) Most of the particles passed directly through - no deflection
2) Some of the particles were deflected somewhat from their path.
3) Some of the particles bounced back in the same direction from which they approached the gold foil.
Thomsonís Model says: all particles should go through and ...
he canít explain the 1/20,000 that deflected
back ...
So, Rutherford proposed his own atomic model:
Conclusions:
1)
____________________________________________________________________
2)
____________________________________________________________________
_______________________________________________________________________
3) ____________________________________________________________________
_______________________________________________________________________
Rutherfordís Atomic Model:
Atoms have a _________________, _______________, which he called the
_____________________.
VI. James Chadwick
An atom contains ________ __________ around a
___________ ___________. Are there any ________
____________ in the atom? In 1932,
_______________ did an experiment to find out.
Observation: Chadwick found that _______changed to _______
Conclusion: ________ ________ came out: called ________
VII. A. Parts of the Atom:
Names:
Size:
Mass present:
Subatomic
Particles
present:
B. Properties of the Three Key Subatomic Particles
Name
Charge
Mass
(Symbol)
Relative Absolute
(C) Relative(amu)
Absolute (g)
+ 1.6 x 10-19
1.67262 x 10-24
0
1.67493 x 10-24
- 1.6 x 10-19
9.10939 x 10-28
The number of ________________ determines what element you have.
This is called the ___________ _________________: the symbol for atomic number is _______
If Z = 14, the atom is _______
For Cl, Z = _______
The total number of protons and neutrons is called the _________
________ : the symbol for the mass number is _______
Carbon, C, has _____ protons and ______ neutrons; A = _______
___________________ is a shorthand way to show what is in a ___________
A
Z X
A = ________________________________________________________
Z = ________________________________________________________
N = A - Z = _________________________________________________
Nuclear Notation:
9 P
10 n =
49 P
66 n =
12
________ protons
C
________ neutrons
6
59
________ proton
Co ________
neutrons
27
128
________ protons
Te
________ neutrons
52
__________________: same__________ different
_________ because of different number of ____________
Three Isotopes of Hydrogen
1
2
3
1 H
1 H
1 H
Name: ____________
____________
______________
______ proton _____ proton
_____ proton
_____ neutrons
_____ neutrons _____ neutrons
Two common Isotopes of Uranium:
238
____
___ U
____ U
______ protons
______ protons
______ neutrons
143 neutrons
VIII. Atomic Mass System
How can we find the mass of one atoms? They are much to small for us to measure - so we develop a relative scale:
Definition: _________________________________________________________
therefore, the mass of 1 H atom is _______,________
(Note: 1 ________ = 1.67 x 10-24 g)
____________ is the standard mass and the mass of all other atoms is measured relative to C
What is the atomic mass of carbon according to the periodic table?
______________________
Why?
The 12.011 amu is the_________ mass of carbon atoms in nature. The number of
________ in the
nucleus of every carbon atom is __________, but the number
__________of can vary. The ________
_____________ of carbon atoms (or atoms of any element) can vary. The most common form of carbon is:
_____________________________________
Use this isotope notation (element name - mass number) to name the
other two naturally occurring isotope of carbon:
_______________
_______________
Example: Determine number of subatomic particles in the isotope
of an element
Silicon (Si) is essential to the computer industry as a major
component of semiconductor chip. It has three
naturally occurring isotopes: Silicon-28, Silicon-29, and
Silicon-30. Determine the number of protons, neutrons,
and electrons in each silicon isotope:
Example: How many protons, neutrons, and electrons are in each of
the following:
(a)
(b)
(c)
What element symbols do Q, X, and Y represent?
Example: Calculating the atomic mass of an
element
Oxygen exists in nature in three forms:
Oxygen-16
Oxygen-17
Oxygen-18
What is the number of protons, neutrons, and electrons in each isotope?
protons neutrons
electrons
Oxygen-16
Oxygen-17
Oxygen-18
Calculate the average atomic mass of oxygen atoms given the following
isotopic data:
ISOTOPE
MASS (amu)
ABUNDANCE
%
Fractional
Oxygen-16
15.99491 amu
Oxygen-17
16.99914 amu
Oxygen-18
17.99916 amu
The ____________ _______________ ________________ of oxygen is:
Oxygen-16
0.99759 (15.99491 amu)
Oxygen-17
+ 0.00037 (16.99914 amu)
Oxygen-18
+ 0.00204 (17.99916 amu)
Example: In nature, copper is found to exist in two forms: copper-63 and copper-65. Copper-63 atoms
have a mass of 62.93 amu, while copper-65 atoms have a mass of 64.93 amu. Naturally occurring copper
contains 69.40% copper-63. Calculate the atomic mass of naturally occurring copper atoms.
IX. The Periodic Table - continued
A. Dmitri Mendeleev 1836 - 1907
- Professor of Chemistry at University of St. Petersburg
- Developed the periodic table while writing a chemistry textbook for
his students
- Based on the observation that when the elements were arranged in order
of their
weight (mass) , similar chemical properties repeated at periodic intervals
- German physicist Julius Lothar Meyer developed similar periodic table
within
the same time period as Mendeleev
B. 1887 - 1915 Henry Moseley
- English physicist
- Discovered that every element had a different number of protons
- Arranged the elements in a table by order of atomic number instead of atomic
mass
C. The Modern Periodic Table
_________- the horizontal rows (identified by a number)
_________- the vertical columns (identified by a number and a letter
Any element can be located with a period number and group number
Na is located in Period _____, Group ______
O is located in Period _____, Group ______
Fe is located in Period _____, Group ______
Group A elements (IA - VIIA) are the ____________________________
Group B elements are the ________________________ ______________
and
_____________ ______________________ ________________
Family names on the Periodic Table:
- Group IA elements are the ___________________
- Group IIA elements are the ___________________
- Group VIIA elements are the ___________________
- Group VIIIA elements are the_________________ or ______________________
|