States of Matter
and Introduction to
Thermochemistry
I. States of Matter
A. Solid
1. Properties
a. has distinct volume and well-defined shape (does not take shape of its container)
b. particles tend to vibrate about fixed points
c. particles are packed against one another in a rigid and organized arrangement
2. Phase changes of solids
a. Melting; Freezing
melting
Solid ----------------> Liquid
<---------------
freezing
_________________ - temperature at which a solid turns into a liquid
_________________ - temperature at which a liquid turns into a solid
The ___________________ and __________________ are the ____________temperature.
_____________________ have ___________ melting points (ex. melting point of NaCl = 801
oC)
_____________________ have ___________ melting points (ex. melting point of hydrogen chloride = -112
oC)
b. Sublimation; Deposition
______________________ - the change of a substance from a solid to a vapor or without passing through the liquid state
______________________ - the change of a substance from a vapor to a solid without passing through the liquid state
sublimation
Solid ------------------> Gas
<-----------------
deposition
Examples of solid that sublimate: _______________________, _______________ (_______________________), __________________
B. Liquid
1. Properties
a. occupies fixed volume, but has no shape of its own
b. particles are relatively free to move and slide past one another
c. particles vibrate and spin as they move from place to place
2. Phase Changes of Liquids
a. Freezing; Melting
b. Vaporization; Condensation
vaporization or evaporation
Liquid --------------------------------------> Vapor (Gas)
<--------------------------------------
condensation
_______________________ - conversion of a liquid to a gas or vapor
__________ - matter that has no definite shape or volume; it is the normal state of the substance at room temperature (ex. oxygen, nitrogen, argon)
____________ - gaseous state of a substance that is normally a liquid or solid at room temperature (ex. water vapor)
__________________ - conversion of a liquid to a gas or vapor occurs at surface of the liquid that is not boiling
__________________ - temperature at which the vapor pressure of a liquid just equals the external pressure
__________________ - boiling point of a liquid at a pressure of 1 atm
C. Gas
1. Properties
a. takes shape and volume of its container
b. particles of a gas move rapidly in constant random motion
c. particles move independent of each other (there is no attractive or repulsive force between the particle)
2. Phase Changes of Gases
a. Condensation; Vaporization (Evaporation)
Condensation
Vapor (Gas) ------------------------------------------> Liquid
<------------------------------------------
Vaporization or Condensation
b. Sublimation; Deposition
Deposition
Gas --------------------> Solid
<-------------------
Sublimation
D. Plasma - the fourth state of matter?
Plasma occurs at extremely high temperatures (millions of degrees C). At these temperatures, all electrons have been removed from the atom. The resulting fluid of bare nuclei and free electrons is called ____________________.
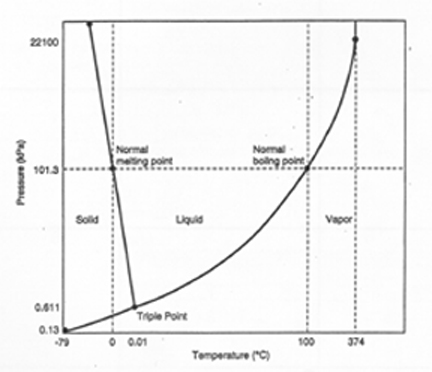
E. Phase Diagrams
1. ____________________ - gives the conditions of temperature and pressure at which a substance exists as solid, liquid, and gas (vapor)
2. Every substance has its own characteristic phase diagram
3. ____________________ - the temperature and pressure under which all three phases of a substance may exist in equilibrium with one another
 |
III. What is THERMOCHEMISTRY and how does it relate to physical and chemical
changes?
A. Introduction to Thermochemistry
1. Definitions of Energy, Work, Heat, Temperature
a. ______________ - the ability (or capacity) of a system to do work or
supply (or produce) heat
b. _________________
simple definition - _______________________________________________
more complex definition - _________________________________________
__________________________________________
c. ________ - transfer of energy between two objects due to
temperature differences
d. ______________________ - property which determines the direction heat will
flow when two objects are brought into contact
2. Thermochemistry
a. Types of processes
_____________________ - process that absorbs heat from the surroundings (q > 0)
_____________________ - process that releases heat to its surroundings (q < 0)
b. Equation that expresses heat changes:
Heat = q =
q = ______________________ (expressed in calories or Joules)
note: 1000 calories = 1 kilocalorie = 1 Calorie
4.184 J = 1 calorie
m = ____________________________ (in grams)
T = ____________________________ (in oC)
C = ___________________________(cal/g oC or J/g oC)
_______________________- the amount of heat it takes to raise the temperature of
1 g of a substance 1 oC
c. Specific heats of some common substances
from Table 11.2, p. 296
 |
d. Thermochemistry problems
Problem #1: How much energy must be absorbed by 20.0 g of water to increase its
temperature from 25.0 oC to 100.0 oC?
Problem #2: How much energy must be absorbed by 20.0 g of iron to increase its
temperature from 25.0 oC to 100.0 oC?
Problem #3: When 15.0 g of steam drops in temperature from 275.0 oC to 250.0 oC?
d. Questions
1) On hot summer days, why is the air cooler near large bodies of water such as
lakes?
2) A puddle of water and a manhole cover sit out on a hot sunny day. Which is
hotter? Why?
3) Explain the following statement by Mark Twain: “The coldest winter I ever
spent was the summer I spent in San Francisco.”
B. How to measure heat changes: Calorimetry
_______________________ - way to measure heat change for a chemical or physical
process
C. Heat Changes in Phase Changes
1. Heat of Fusion and Solidification
______________________________ ( ^ Hfus) - heat absorbed by one mole of a
substance in melting from a solid to a liquid at a constant temperature
______________________________ ( ^ Hsolid) - heat lost when one mole of a liquid
solidifies at a constant temperature
^
Hfus = ^ Hsolid
Example: water
H2O (s) ---> H2O (l)
^ Hfus = 6.01 kJ/mol
H2O (l) ---> H2O (s)
^ Hsolid = - 6.01 kJ/mol
Molar Heat of Fusion Equation: q = ^ Hfus x moles
q = ^ Hfus x mass
x 1
molar mass
Problem #1: 3.14 grams of water is being melted at its melting point of
0 oC. How much energy (in kJ) is required?
Problem #2: 53.1 grams of water exists as a liquid at 0 oC. How much
energy must be removed to turn the water into a solid at 0 oC?
Questions:
1) At what temperature does water freeze?
2) At what temperature does ice melt?
3) In freezing weather, how does spraying fruit and flooding orchards with a few
inches of water keep the fruit from freezing?
2. Heat of Vaporization and Condensation
________________________________ ( ^ Hvapor) - the amount of heat necessary to
vaporize (boil) one mole of a liquid
________________________________ ( ^ Hcond ) - the amount of heat released when
one mole of a vapor condenses
^
Hvapor = - ^ Hcond
Example: water
H2O (l) ---> H2O (g)
^ Hvapor = 40.7 kJ/mol
H2O (g) ---> H2O (l)
^ Hcond = - 40.7 kJ/mol
Molar Heat of Vaporization Equation:
q = ^ Hvapor x moles
q = ^ Hvapor x mass x 1
molar mass
Problem #1: 49.5 g of water is being boiled at its boiling point of 100 oC. How
many kJ is required?
Problem #2: 80.1 g of water exists as a gas at 100 oC. How many kJ must be
removed to turn the water into liquid at 100 oC?
More Practice:
Problem #3: How many grams of ice at 0 oC could be melted by the addition of
2.25 kJ of heat?
Problem #4: How many kilojoules of heat are required to melt a 10.0 g popsicle
at 0 oC? Assume the popsicle has the same molar mass and heat capacity as water.
Problem #5: How much heat (in kJ) is absorbed when 63.7 g of liquid water at 100
oC is converted to steam at 100 oC?
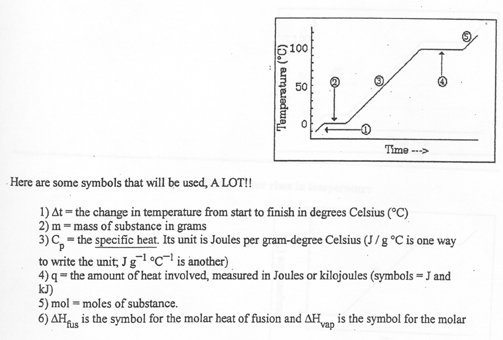
D. Time-Temperature Graph
Problem: Heat a container that has 72.0 grams of ice from -10.0 oC to 120.0
oC.
Five major steps are required to solve this problem:
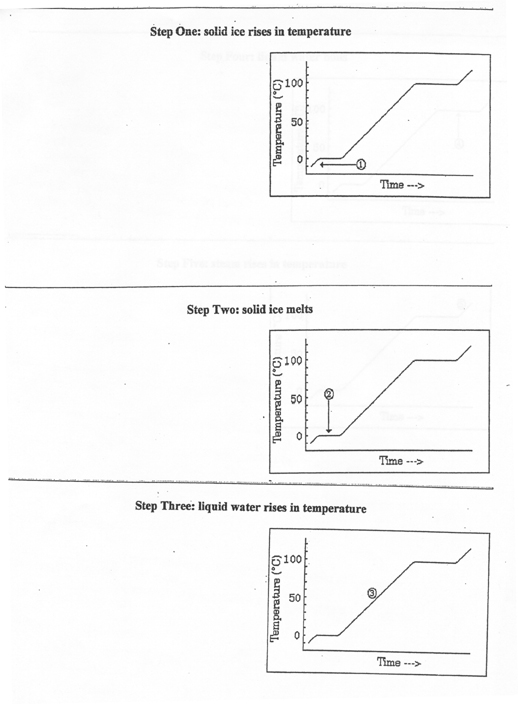
1) the ice rises in temperature from -10.0 oC to 0.0 oC
2) the ice melts at 0.0 oC
3) the liquid water then rises in temperature from zero to 100.0 oC
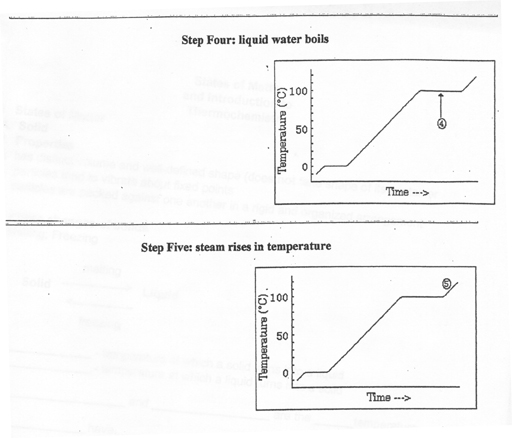
4) the liquid water then boils at 100.0 oC
5) the steam then rises in temperature from 100.0 oC to 120.0 oC
Each step requires a calculation.
 |
 |
 |
| HOMEPAGE | |
II. What is THERMOCHEMISTRY and how does it relate to physical and chemical changes?
A. Introduction to Thermochemistry
1. Definitions of Energy, Work, Heat, Temperature
a. ______________ - the ability (or capacity) of a system to do work or supply (or produce) heat
b. _________________
simple definition - _______________________________________________
more complex definition - _________________________________________
_________________________________________ c. ________ - transfer of energy between two objects due to temperature differences
d. ______________________ - property which determines the direction heat will flow when two objects are brought into contact
2. Thermochemistry
a. Types of processes
_____________________ - process that absorbs heat from the surroundings (q > 0)
_____________________ - process that releases heat to its surroundings (q < 0)
b. Equation that expresses heat changes:
Heat = q =
q = ______________________ (expressed in calories or Joules)
note: 1000 calories = 1 kilocalorie = 1 Calorie
4.184 J = 1 calorie
m = ____________________________ (in grams)
T = ____________________________ (in oC)
C = ___________________________(cal/g oC or J/g oC)
_______________________- the amount of heat it takes to raise the temperature of 1 g of a substance 1 oC
c. Specific heats of some common substances
from Table 11.2, p. 296
d. Thermochemistry problems
Problem #1: How much energy must be absorbed by 20.0 g of water to increase its temperature from 25.0 oC to 100.0 oC?
Problem #2: How much energy must be absorbed by 20.0 g of iron to increase its temperature from 25.0 oC to 100.0 oC?
Problem #3: When 15.0 g of steam drops in temperature from 275.0 oC to 250.0 oC?
d. Questions
1) On hot summer days, why is the air cooler near large bodies of water such as lakes?
2) A puddle of water and a manhole cover sit out on a hot sunny day. Which is hotter? Why?
3) Explain the following statement by Mark Twain: “The coldest winter I ever spent was the summer I spent in San Francisco.”
B. How to measure heat changes: Calorimetry
_______________________ - way to measure heat change for a chemical or physical process
C. Heat Changes in Phase Changes
1. Heat of Fusion and Solidification
______________________________ ( Hfus) - heat absorbed by one mole of a substance in melting from a solid to a liquid at a constant temperature
______________________________ ( Hsolid) - heat lost when one mole of a liquid solidifies at a constant temperature
Hfus = Hsolid
Example: water
H2O (s) ---> H2O (l) Hfus = 6.01 kJ/mol
H2O (l) ---> H2O (s) Hsolid = - 6.01 kJ/mol
Molar Heat of Fusion Equation: q = Hfus x moles
q = Hfus x mass x 1
molar mass
Problem #1: 3.14 grams of water is being melted at its melting point of
0 oC. How much energy (in kJ) is required?
Problem #2: 53.1 grams of water exists as a liquid at 0 oC. How much
energy must be removed to turn the water into a solid at 0 oC?
Questions:
1) At what temperature does water freeze?
2) At what temperature does ice melt?
3) In freezing weather, how does spraying fruit and flooding orchards with a few inches of water keep the fruit from freezing?
2. Heat of Vaporization and Condensation
________________________________ ( Hvapor) - the amount of heat necessary to vaporize (boil) one mole of a liquid
________________________________ ( Hcond ) - the amount of heat released when one mole of a vapor condenses
Hvapor = - Hcond
Example: water
H2O (l) ---> H2O (g) Hvapor = 40.7 kJ/mol
H2O (g) ---> H2O (l) Hcond = - 40.7 kJ/mol
Molar Heat of Vaporization Equation:
q = Hvapor x moles
q = Hvapor x mass x 1
molar mass
Problem #1: 49.5 g of water is being boiled at its boiling point of 100 oC. How many kJ is required?
Problem #2: 80.1 g of water exists as a gas at 100 oC. How many kJ must be removed to turn the water into liquid at 100 oC?
More Practice:
Problem #3: How many grams of ice at 0 oC could be melted by the addition of 2.25 kJ of heat?
Problem #4: How many kilojoules of heat are required to melt a 10.0 g popsicle at 0 oC? Assume the popsicle has the same molar mass and heat capacity as water.
Problem #5: How much heat (in kJ) is absorbed when 63.7 g of liquid water at 100 oC is converted to steam at 100 oC?
D. Time-Temperature Graph
Problem: Heat a container that has 72.0 grams of ice from -10.0 oC to 120.0 oC.
Five major steps are required to solve this problem:
1) the ice rises in temperature from -10.0 oC to 0.0 oC
2) the ice melts at 0.0 oC
3) the liquid water then rises in temperature from zero to 100.0 oC
4) the liquid water then boils at 100.0 oC
5) the steam then rises in temperature from 100.0 oC to 120.0 oC
Each step requires a calculation.