I. Models of the Atoms: Review and New (Read Section 13.1)
A. Dalton model (1803)
B. Thomson model (1897)
C. Rutherford model (1911)
D. Bohr model (1913)
E. Quantum mechanical model (1926)
II. Behavior of Excited Atoms (Read Section 13.3)
A. Nature of Light
1. Wave nature of light
a. Parts of a wave
_________________
_________________ - high point
_________________ - low point
________________________ - wave’s height from the origin to the top of the crest (or
bottom
of the trough)
________________________ - distance from the top of one crest to the top of the next
crest (or...)
b. Two variables that describe a wave
____________________ _______
(the Greek letter lambda)
units: __________________ or ___________________
1 A = 1 x 10 -10 m
___________________ (the Greek letter "nu")
Def'n: the number of wave cycles to pass a given point per unit time
units: ______________________________ or
__________________________
1 Hz = 1 (cycle) / second where “cycle” is understood and not explicitly
stated
= 1 / sec
= sec-1 = s-1
More:
AM radio waves broadcast in kHz (1 x 103 / sec or 1 x 103 sec-1)
FM radio waves broadcast in MHz (1 x 106/ sec or 1 x 106 sec-1)
_________________ = ________________ x ________________
= ________________ x ________________ =
2. Light is __________ part of the
Electromagnetic spectrum
_______________________ ______________ - includes every different kind of energy that:
1) has no mass
2) travels at the speed of light
3) can travel through a vacuum
4) exists in "packets" called _________________
Light, Radio waves (and all other electromagnetic radiation) travel at:
___________________________ ( c ) = 3.00 x 108 m/s
is a _______________
so: c =
Since c= constant, then _______________ = constant. What does that tell us about the
relationship between_________
and___________ ?
Practice Problem: Interconverting wavelength and frequency
An AM radio wave broadcasts music at “960 on your radio dial”. Units for AM
frequencies are given in kilohertz (kHz). Find the wavelength of these radio
waves in m, nm, and Angstroms. Assume the waves travel at the speed of all
electromagnetic radiation 3.00 x 108 m/s.
2. Light is one part of the Electromagnetic Spectrum
____________________ _________________ - continuum of radiant energy
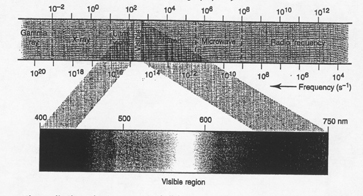 |
Questions:
1) Name the types of electromagnetic radiation that are not visible.
2) Which of these invisible ray have more energy than visible light?
Explain.
3) What do all the rays in the electromagnetic spectrum have in common?
4) Which color of light has a longer wavelength, yellow or blue?
5) What kind of energy is also known as thermal energy?
6) How does the speed of microwaves compare with the speed of x-rays?
More Practice: Interconverting wavelength and frequency
A dental hygienist uses x-rays ( = 1.00 A ) to take a series of dental
radiographs while the patient
listens to a radio station ( = 325 cm) and looks out the window at the blue sky ( = 473 nm).
What is the frequency (in s-1) of the electromagnetic radiation from each source? What station is the
radio broadcasting at?
3. Particle nature of light
a. Two of the phenomena involving matter and energy that could not be
explained by the wave nature of light in the early 1900’s:
1. _____________________ - the flow of current when ____________________
light of sufficient energy shines on a metal plate
________________________ - light of a single wavelength
________________________ - light of many wavelengths
Is sunlight monochromatic or polychromatic?
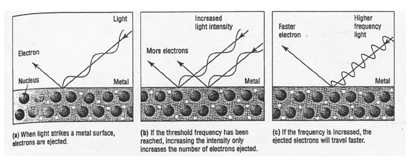 |
Examples of the photoelectric effect: _________________________
2. Atomic Emission Spectrum
b. To describe the particle nature of light:
_____________________ - “particle” or “packet” of light; light ___________________
_____________________ - fixed quantity; plural, quanta
Ephoton =
Ephoton =
h = _____________________ = 6.626 x 10-34 J . s
= frequency
Question: Why don’t we see “separate packets” or photons of light coming
from a light bulb?
Practice Problem: calculate the energy of radiation from its frequency
1. Calculate the energy (in J) of a quantum of radiant energy (the energy of
a photon) with a frequency of 5.00 x 1015 sec-1?
2. The threshold photoelectric effect in tungsten is produced by light of
wavelength 260 nm. Give the energy of a photon of this light in joules.
3. A cook uses a microwave oven to heat a meal. The wavelength of the
radiation is 12.0 cm. What is the energy of one photon of this microwave
radiation?
B. Types of Spectra
1. ______________________ spectra - “rainbow” of colors
Visible light is a _______________________ spectrum
1) List the colors of the visible spectrum in order of increasing
wavelength.
2) What is the range of wavelengths from the electromagnetic spectrum that
represents visible light?
2. _____________________ spectra - series of fine lines of individual colors
separated by
colorless (black) spaces
For an “excited” atom:
_______________________ - electrons of an atom are not in there ground state
For example: when electricity is discharged through the gas or vapor of an
element
1) _________________________________________________________________________________________
2) _________________________________________________________________________________________
3) _________________________________________________________________________________________
4) _________________________________________________________________________________________
Passing this light through a diffraction grating gives the
_______________________________ of the element.
Example: the atomic emission spectrum of hydrogen is a ____________________________
spectrum
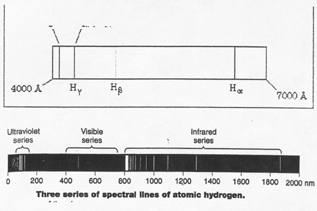 |
There are______________________ ______________________ ____________________ in the hydrogen spectrum.
1) there is one ________________________ line at _______________
2) there is one ________________________line at ________________
3) there is one ________________________ line at _______________
4) there is one ________________________ line at _______________
This series of visible lines is called the _______________________. This series
is due to the transition of electrons from higher energy levels to the
___________ energy level.
There are two other series associated with the Hydrogen spectrum that are
not visible.
The ______________________ corresponds to the transition of high energy “excited”
electrons to ____________energy level. These lines are in the
____________________spectrum.
The ______________________ correspond to the transition of “excited” electrons to
the _____________________ level. These lines are the ____________ spectrum.
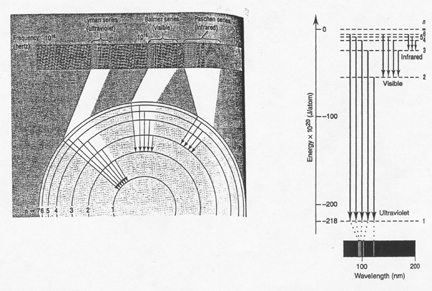 |
Every element has a ________________ ______________ __________________
____________________ that can be used to identify the elements.
Question: How was helium discovered?
III. Back to Bohr and more...
A. The Bohr Model of the Atom
1. The Model
2. Bohr’s ideas:
1) _______________________________________________ (this was Rutherford’s discovery)
2) _______________________________________________________________________________________;
the electrons have a fixed energy and do not lose energy while in this
orbit; these orbits are referred to as energy levels
3) when an electron moves from one energy level to another,
________________________________________________________________________________________________________________________________________
4)______________________________________________________________________________________________________________________________________________;energy
levels become more closely spaced the farther the distance from the nucleus;
it takes less energy for an electron to escape the further away the electron
is from the nucleus;
5)
____________________________________________________________________________________________________________________________________
3. The problems with Bohr’s model:
1) _________________________________; it failed to predict the spectrum of
any other atom other than H
2) ________________________________________________________________
but ... it still holds that
1) _______________________________________________________________
2) we still use the terms ____________________________ and ___________________________
B. The Quantum Mechanical Model
Erwin Schrodinger (1926)
Used Bohr’s ideas to derive a mathematical equation which describes the
location and energy of electrons
describes an atom that has certain quantities of energy
electrons have wavelike properties
atomic orbitals are __________________________________ where the probability of
finding the electrons are high
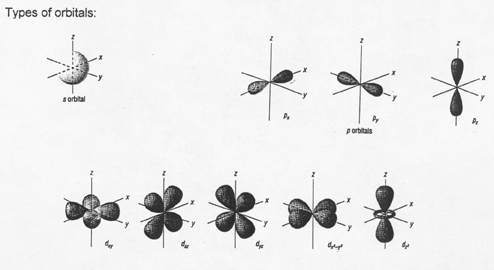
Types of orbitals:
s p d
 |
C. Where do the electrons go?
1. The Energy Levels
n = ______________________________
indicates the ________________________ of the orbital and the
___________________________________
the ___________________ the n value, the ____________ the ____________
Example: for the H atom in the ground state, its electron is in the n=1
level
2. The Sublevels, Orbitals, and Spin
the ________________________ are the ____________shapes (“speedy
frank”)
each __________________ has _______ orbital (each s sublevel can hold _____
electrons)
each __________________ has _______ orbitals (each p sublevel can hold
_____electrons)
each __________________ has _______ orbitals (each d sublevel can hold _____
electrons)
each __________________ has _______ orbitals (each f sublevel can hold ______
electrons)
 |
_______________________________________________________; each with opposite spin
3. The Energy Levels; Atomic Orbitals; Electrons for n=1 to n=4
Energy Level Sublevels Maximum Number of e-
What is the maximum number of electrons that can be found in any energy
level?
__________________________________________________________________
4. Review of Energy Levels, Sublevel, and Orbitals
a. Energy levels are designated by the letter _______ and are
numbered ________ .
__________________ is ________________to the nucleus.
__________________ is ________________ from the nucleus.
b. Each energy level contains as many sublevels as the number of the energy
level.
Ex. When __________, there are ____ sublevels: _____ and _____ .
c. Each energy level contains __________ orbitals.
Ex. When ______ , there are ______ orbitals
Ex. When _________ , there are ______ sublevels (3s, 3p, 3d) and
________ orbitals.
d. Since each orbital contains ________ electrons, the maximum number of
electrons that an energy level can hold is _________ .
Ex. When _________, there are _____ electrons in the second energy
level.
Ex. When ________ , there are sublevels
there are n2 or orbitals
there are 2n2 or electrons
5. How do electrons fill these energy levels?
a. Follow three rules:
1) __________________________: Electrons enter the lowest energy orbital
first.
2) _________________________________________: An atomic orbital can only
hold two electrons.
3) ________________________: No electron pairing takes place until each
orbital of a given set contains one electron.
b. Order of filling sublevels
1s
2s 2p
3s 3p 3d
4s 4p 4d 4f
5s 5p 5d 5f 5g
6s 6p 6d 6f 6g
7s 7p
c. Aufbau Orbital Diagram
Aufbau Orbital Diagram Electron Configuration
H: _____
1s
He: _____
1s
Li: _____ _____
1s 2s
Be: _____ _____
1s 2s
Example:
Write the Aufbau orbital diagram and the electron configuration for the
element potassium.
e. Shorthand notation
Ex. Write the long form of electron configuration and the shorthand
configuration of calcium.